Abstract
Introduction: Mantle cell lymphoma (MCL) is a non-Hodgkin lymphoma representing 4-9% of all lymphomas. The disease prognosis remains adverse. However, diagnosis and treatment of MCL have undergone a significant improvement in the last years.
The World Bank considers Mexico as a Middle-Income Country (MIC). Therefore, MICs experience a delay in accomplishing diagnosis and prognosis strategies and low access to treatment innovation for hematologic malignancies. In Mexico, there is scarce information on the clinical features and treatment patterns of MCL over time. Mainly, the prognosis impact of these strategies is unknown.
Methods: We retrospectively evaluated all consecutive patients with a pathological diagnosis of MCL in three referral centers for the uninsured population in Mexico from 2008 to 2020. We followed patients to June 2021 until lost follow-up or death. We made a Time-Dependent-Analysis (TDA) to evaluate the effect of the diagnosis and treatment improvements over time. We divided the population into two groups, from 2008 to 2014 (Group A) and from 2015 to 2020 (Group B). Hematopathologists reviewed all cases at their respective centers. We excluded patients that do not receive treatment and patients without pathological confirmation. Clinical, pathological, and treatment data were collected. Responses were assessed per the Lugano criteria. Event-free survival (EFS) and overall survival (OS) were estimated using the Kaplan-Meier method, differences between the groups were evaluated with a log-rank test. Multivariate analysis of factors associated with mortality was assessed with a Cox regression model with a Confidence Interval(CI) of 95%.
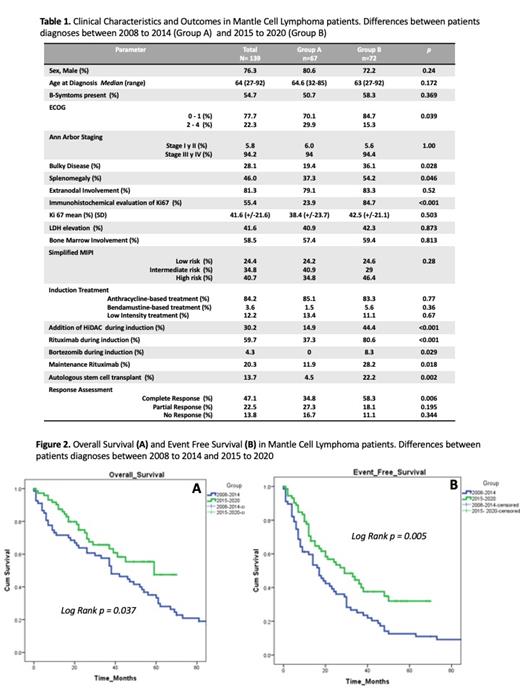
Results: A total of 139 patients were included in the analysis; 67 patients from Group A; and 72 patients from Group B. The median age was 64 years, 42% were younger than 65 years, 76% were male, 22% had ECOG 2-4, 81% had extra nodal involvement, 28.1% had bulky disease, 94% had stage III/IV disease, 58% had bone marrow involvement, 40.7% had high risk simplified MIPI. Regarding diagnosis approaches between the two groups, only 23% in Group A had an immunohistochemical evaluation of Ki67 compared with an 84% of Group B (p<0.001). The rest of the differences between the groups are in Table 1.
In terms of treatment, 84% received an anthracycline-based treatment, 3% bendamustine-based treatment, and 12% a low-intensity treatment, with no difference between the groups. In addition to base treatment, 30% received High-dose Cytarabine (HiDAC), been more frequent in Group B (p<0.001), 59% received rituximab during induction, 37% in Group A, and 80% in Group B, (p<0.001). The main reason to avoid the use of rituximab was the cost and only 20.3% used rituximab maintenance, being more frequent in Group B (p=0.018). The 13.7% received an autologous stem cell transplant, been more frequent in Group B (p= 0.002). Only two patients (1.4%) received treatment in a Clinical Trial setting. In terms of response, in Group A, 58% achieved Complete Response (CR) against 34% of Group B (p=0.006).
Median OS on Group A was 38 months (95% CI 25.6 -50.3) against Not Reached in Group B (Log Rank p = 0.037). On the other hand, the median EFS on Group A was 17 months (95% CI 11.6-22.39) against 29 months (95% CI17.6-40.3) in Group B (Log Rank p = 0.005) Figure 2. A multivariate analysis of factors associated with higher mortality found a high-risk MIPI score with an HR of 2.54 (CI 95% 1.5-4.0, p <0.001). Factors associated with lower mortality were the addition of HiDAC in induction HR 0.39 (CI 95% 0.20- 0.75, p= 0.005) and rituximab maintenance HR 0.48 (CI 95% 0.24-0.94, p=0.035)
Conclusion: This is the first real-world report of MCL in Mexico. Characteristics of the disease resemble the ones reported by other countries. Despite the limitations of a retrospective analysis, the TDA makes evident the prognosis improvement over time. With progress in EFS of 12 months and an OS impact. Even though the prognosis improvement, to the date, there is a 20% of patients with no rituximab access. Transplant access is low beside that 42% of patients were younger than 65 years, and Clinical Trial access is almost null. Novel therapies for MIC are under development with encouraging results in the first-line and relapsed/refractory setting. However, the high costs of these therapies will limit the access in MIC and would expand the gap of MCL prognosis if a health care strategy that allow access to them is not implemented.
Rangel-Patiño: Abbvie: Speakers Bureau; Bristol: Consultancy. Agreda: Roche: Speakers Bureau; Astrofarma: Speakers Bureau; Janssen: Speakers Bureau. Gomez-Almaguer: Bristol-Myers-Squibb: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Roche: Honoraria, Speakers Bureau. Ramirez-Ibarguen: Takeda: Consultancy, Speakers Bureau; Roche: Speakers Bureau; Janssen: Speakers Bureau; Astra Zeneca: Speakers Bureau; Abbvie: Speakers Bureau; MSD: Consultancy; Asofarma: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal